The Formula for Potassium Sulfide Is Therefore
The molecule is formed by two potassium cation K and one sulfide anions S 2-. It is considered as an ionic compound.

Solved 7 1 Point What Is The Formula Of Potassium Sulfide Chegg Com
Cations have positive electrical charge whereas anions have.
. Therefore there must be two k ions for every oxygen 2- ion. Name the following compound. K 2 S 2 O 5 K 2 SO 3 SO 2.
It has the chemical formula S 2. Find an answer to your question What is the correct formula for potassium sulfite. The formula for potassium oxide is K2O.
Following this image we know that since potassium has just 1 positive charge then there is just 1 molecule of sulfate. Atoms or even atom groups with electrical charge. The potassium sulfide chemical formula is K 2 S.
For an electrically neutral ionic compound the charges must balance. Sulphur is a chemical compound with the symbol S and has the atomic number 16. The structural formula of Potassium Sulfite is as shown in the figure below.
Thus the right option is B. The chemical formula of potassium sulfate is rmK_2rmSrmO_4. Potassium sulfate can be represented as.
Cations and anions are two classes of ions ie. A sulfide ion has a charge of 2-. Therefore the formula is K2S.
It contains potassium left rmK right and sulfate left rmSrmO_42 right ions. 2K S 2 K 2 S Therefore the. A potassium ion has a charge of 1.
Potassium Sulfite Structural Formula. The potassium sulfite is formed by the decomposition of potassium metabisulfite at 190C. The molar mass is 11026 gmol.
Potassium sulfate also known as arcanite or sulfate of potash is an inorganic salt that is mostly used as fertilizer. The stock system name for Mn 2 O 7 is a Dimanganese heptaoxide b Magnesium oxide c ManganeseVII oxide d ManganeseII. Potassium sulfide is an ionic compound that can hydrolyze in water to give.
The formula for sodium sulfide is a NaS b K 2 S c NaS 2 d Na 2 S e SeS. The geometry of the molecule is an antifluorite structure with a cation K being surrounded by eight sulfide anions. Potassium sulfide i put KaS K is potassium and it is 1 while sulfide is -2.
We need to equalize charges by criss-crossing the charges like this example. Therefore it needs 2 potassium that adds to a charge of 2 and therefore resulting to the formula K2S. In this video well write the correct formula for Potassium sulfide K2STo write the formula for Potassium sulfide well use the Periodic Table and follow.
Sulphide is an inorganic anion of sulphur. The formula for Potassium Sulfide is K2S therefore the molecular weight of Potassium Sulfide is 39 2 32 110. In order to balance the total charge of 2 on the sulfide ion two potassium ions must combine with it in a 2.
The formula for Potassium Sulfide is K2S therefore the molecular weight of Potassium Sulfide is 39 2 32 110. For more such interesting information along with videos subscribe BYJUS. Potassium sulfide K2S Kaliumsulfid Potassium monosulfide Dipotassium monosulfide Potassium sulfide 21 More Molecular Weight.
Potassium is a highly reactive metal as it has only 1 electron in its valence shell and is placed in group 1 so it has a group oxidation state of 1. Visit BYJUS to learn more about the. Therefore one mole of.
The potassium sulfate chemical formula is K 2 SO 4 and its molar mass is 174259 gmol. Ions that are made of more than 1. Therefore one mole of.
What is the chemical formula for potassium sulfide. Sulphur is sometimes found in the pure form but mostly occurs as sulphide or minerals of sulfate. While sulfur is a non metal that is placed in group 16 and has a group oxidation state of 2 and 4.
The two ions are bound trough ionic bond. This would result in having TWO potassium so potassium would have a charge of 2 neutralizing with sulfurs -2 charge. Potassium Sulfate formula also known as Dipotassium sulfate formula or Sulphate Of Potash formula is an inorganic compound consists of one sulfur atom four oxygen atoms and two potassium atoms.
Although nonmetals may bond in different combinations there is only one combination of a particular pa See Hint neutral compound When forming a monatomic ion K wil The formula for potassium sulfide is therefore electrons while S will electrons. Answer 1 of 5. Both potassium chloride and potassium sulfide are made of two different things.
Hence the formula for potassium sulfide is K_2S. We cant just combine them as it is since potassium and sulfate have different charges. The potassium ion has a charge of 1 and the oxygen ion has a charge of 2-.
Khso khso4 k2so4 k2so3. Therefore its salt with potassium ion K that is KHCO 3 is named as potassium hydrogen carbonate. 1 ratio as follows.
Tin IV chloride i put SnCl4 OK Dihydrogen sulfide i put H2S OK. An atom or group of atoms that has an electric charge. However it needs to neutralize and have a charge of 0 for it to bond successfully.
K 1015 9 OF.
Potassium Sulfide K2s Chemspider
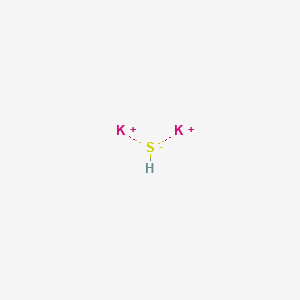

No comments for "The Formula for Potassium Sulfide Is Therefore"
Post a Comment